Application Of Coordination Compounds
- Application Of Coordination Compounds Class 12
- Application Of Coordination Compounds
- Application Of Coordination Compounds In Biological System
- Coordination Compounds Chemistry
- How To Name Coordination Compounds
- Application Of Coordination Compounds In Laboratory
Importance and Application of Coordination Compounds: EDTA is used in the estimation of Ca 2+ and Mg 2+ in hardwater. The Ca 2+ and Mg 2+ ions form stable complexes with EDTA.; Metals can be purified by the formation and subsequent decomposition of their coordination compounds. This review provides an overview of the applicability range of chiroptical methods as a principle tool for chirality investigation, and typical applications of chiral coordination chemistry. The potential of electronic and vibrational chiroptical methods in the design and characterization of coordination compounds is discussed. Coordination Compounds for Medicine and Biology The interaction of transition metal ions with biological molecules provides one of the most fas-cinating areas of coordination chemistry. The application of this field to biomedical uses is dealt with in 5 chapters. Chapter 18 by N. Farrell, deals with the use of metal complexes as drugs. 70 Chapter 6 Applications of Coordination Compounds The sections and subsections in this chapter are listed below. 6.1 Applications of Monodentate Complexes 6.2 Two Keys to the Stability of Transition Metal Complexes Hard and Soft Acids and Bases The Chelate Effect 6.3 Applications of Multidentate Complexes 6.4 Chelating Agents as Detergent Builders.
1.The coordinate covalent or dative bond applies 2.Lewis bases are called LIGANDS—all serve as σ-donors some are π-donors as well, and some are π-acceptors 3. Specific coordination number and geometries depend on metal and number of d-electrons 4. HSAB theory useful a) Hard bases stabilize high oxidation states b) Soft bases stabilize low oxidation.
These are chemistry notes for Coordination Compounds Class 12. For more chemistry classes notes, visit our page or category 12 Class Chemistry Notes.
Coordination Compounds Class 12 Chemistry Notes
Introduction to Coordination Chemistry or Coordination Compounds
Transition metal possessescharacteristic property of undergoing complex formation. Due to this remarkabletendency, they form a large number of coordination compound. The tendency toform coordination compound of several non – transition metals much less.
- Coordination compounds are the compounds in which the centralmetal atom is linked to a number of ions or neutral molecules by coordinatebond.
- Coordinate bond is a covalent type bond in which both theshared pair of electrons are contributed by one atom.
Coordinate compounds are widely distributed in minerals, plants and animals and are known to play many important biological functions.
Coordination Compounds Examples: For example chlorophyll is coordination compound of magnesium and it is essential in photosynthesis. Red pigment hemoglobin is a coordination compound iron.
The branch of chemistry which deals with the study of coordination compounds or complex compound is known as coordination chemistry.
WERNER’S THEORY OF COORDINATION COMPOUND
Basic postulates of Werner’s theory
- Most of the metallic elements exhibit two type of valence;
- Primary valence or principal valence.
- Secondary valence.
- Every metal tends to satisfy both of its primary and secondary valences.
- Every metal has a fixed number of secondary valences.
- The secondary valence is always directed towards fixed position in space.
Werner’s theoryin the light of modern electronic theory of valence.
- According to Werner, two spheres of attraction are present around the metal. The inner zone is coordination sphere and the outer zone is ionization sphere.
- In terms of modern electronic theory of valence, the coordination sphere is equivalent to the coordination number of the metal ion and the ionization sphere is equivalent to the ionisable or the oxidation state of the metal ion.
- Werner introduced a square bracket [ ] to enclose the central metal ion and the ligands. This represents coordination sphere. The ions of the ionization sphere are placed outside the square bracket. e.g. [CO(NH3)6]Cl3, in this complex the coordination sphere includes six ammonia molecules while ionization sphere includes three chloride ions.
Application of Werner’s theory to CO (III) amines in Coordination Compounds Class 12
CO (III) amines are the complex of CO(III) with ammonia. They have compositions.
- [CO(NH3)5]Cl2
- [CO(NH3)5Cl]Cl2
- [CO(NH3)4Cl2]Cl
- [CO(NH3)3Cl3]
Experimentalobservation to CO (III) amines
- The primary valence (or oxidation state) of cobalt is 3 while secondary valence (or coordination number) is 6.
- When cobalt amines are heated with hydrochloric acid at 373 K ammonia is not removed (evolved). In all these compounds ammonia molecules are seen to be firmly bound.
- All these compounds are found to differ in their electrical properties.
- All these compounds are differing in their reactivity towards silver nitrate.
LIGANDS
The molecules or ions which arecoordinated to the central atom or ion in the coordination compound are called ligands.
e.g. i. In [ Ni(NH3)6]2+ ,central metal ion is Ni2+ and ligands are NH3 molecules.
ii. In [Cr(H2O)6]3+ , the central metal ionis Cr3+ and ligands are H2O molecules.
Types of Ligands
Types of ligands and classification of ligands is as follows.
1. Mono or unidentate ligands
The ligand molecules or ion which has only one donor atomor one point attachment and can coordinate with the metal ion at only one sitein a complex is called unidentate ormonodentate ligands.
Onekey recovery download windows 10. Sep 10, 2019 What Is Lenovo OneKey Recovery Windows 10? Lenovo OneKey Recovery is a one key recovery software snap in Lenovo products, desktop, laptop, notebook, notepad, etc. When you get a new computer and have system installed, you can use it to make a recovery partition for future factory format. If your new pc comes to you with os already installed. Download Now! You can access OneKey Recovery from Windows or outside Windows: In Windows, double-click the app icon to start Lenovo OneKey Recovery system; Outside Windows (when system failure occurs and unable to enter system), press NOVO button (either a small button or pinhole with a backwards arrow icon next to it resembing an upside down U).
e.g. Cl– , OH– , NH3 , H2Oetc.
2. Poly multidentate ligands
Theligand molecule or ion in which has two or more donor atom or points ofattachment and can be linked to the same metal in a complex using two or moredonating sites is called poly ormultidentate ligands.
Dependingupon the number of donor atoms, they are further classified as
- Bidentate ligands
e.g. ethylene diammine
- Tridentate ligands
e.g. diethylene triammine
- Tetrodentate ligands
e.g. triethylenetetrammine
Fingerprint drivers for windows 10. Oct 29, 2018 LENOVO X61 BIOMETRIC COPROCESSOR DRIVERS FOR WINDOWS 10 October 29, 2018 admin And Man, and the IP address of your browsing computer is on this list, which found Grays testimony was unreliable and the evidence against Giddens insufficient. If you still need help, post a screenshot of what you see at those steps.
- Hexadentate ligands
e.g.ethylenediamminetetracetate (EDTA)
3. Ambidentate ligands
Ambidentate ligands are the ligands which have two ormore donor atoms capable of forming coordinate bonds; however only one donoratom is utilized during complex formation.
e.g. NO2 group has donor atoms N and O out of the two only one donor atom is linked to the two only one donor atom is linked to the metal as M-ONO or M- NO2.
COORDINATIONNUMBER (CN)
The number of ligands which aredirectly bonded to central metal atom or ion in a complex (coordinationcompound), is known as coordinationmetal of the metal atom or ion. It is abbreviated by symbol ‘CN’.
Incase of metal chalets (complex with polydentate ligands and ring structures).CN is the number of pair electron involved in formation of bonds between metaland ligands. CN is characteristic property of metal. Geometry and shape ofcoordination compound is governed by CN.
Metal ionand coordination number
| Metal ion | Oxidation state | CN | Examples |
| Ag+ | +1 | 2 | [Ag (NH3)2]+ |
| Cu2+, Ni2+, Zn2+, Cd2+, Hg2+, Pt2+ | +2 | 4 | [Cu(NH3)4]2+ |
| Fe3+, CO3+, Cr3+ | +3 | 6 | [CO(NH3)6]3+ |
| Sn4+, Pt4+ | +4 | 6 | [Pt(NH3)6]4+ |
| MO4+ | +4 | 8 | [MO(CN)8]4- |
Thecoordination number of the metal ion is influenced by –
1. Charge on the metal ion.
2. Charge on the ligand.
3. The relative sizes of metal ionand ligands.
4. The forces of repulsion betweenthe ligands etc.
Complex ion: A complex ion is more or less stable,charged aggregate formed. When an ion, mostly of a metal is directly linked toa group of neutral molecules or ions.
Coordinationentity : Acoordination entity constitutes central metal atom or ion bonded to fixednumber of ions or molecules.
Coordinationsphere : Thecentral metal atom or ion and the coordinating group (ligands) attached to itare written inside the square bracket and is together called as coordinationsphere.
Homoleptic andheteroptic complexs
A] Complexes in which a metal isattached to one kind of donor groups are homoleptic.
B] Complexes in which a metal isbound to more than one kind of donor groups are heteroleptic.
Cationic,Anionic and Neutral complexes
A] Cationic complex
A complex in which the complex ion carries a net positive charge is called cationic complex.
e.g. [CO(NH3)6]Cl3
B] Anionic complex
The complexes in which the complex ion carries a net negative charge are called anionic complexes.
e.g. K2[HgI4]
C] Neutral complex
A complex carrying no net charge is called a neutral complex.
e.g. [Ni(CO)4]
Chargenumber of complex ion
The net charge carried by complex ionis called charge number. It is algebraic sum of the charges carried by the centralmetal ion and ligands attached to it.
e.g. charge number of [Fe(CN)6]4-
= charge on Fe2+ + 6 x charge on CN– ion
= (+2) + 6(-1) = -4
Coordinationpolyhedron
The spital arrangement of the ligandatoms, which are directly attached to the central atom / ion is called coordination polyhedron. The commoncoordination polyhedral are tetrahedral, square planner and octahedral.
Double saltsand coordination compounds
Double salts : Double salts are molecular or addition compounds which exist in the solid state but dissociate into their constituents ion when dissolved in water.
Coordination Compounds : Coordination compounds are molecular or addition compounds which retain their identity in aqueous solution and show properties entirely different from those of their constituent ions.
Characteristicsof complex ions
- Generally a transition metal ion is the central metal ion ina complex.
- An ion loses its individual properties and acquires theproperties of the complex ion, which it forms.
- The complex ion can dissociate to a slight extent in thesolution however it retains its identity.
- The algebraic sum of the charges of constituent ion is thenet charge of complex ion.
- The stability of chelate complex is higher than thosecomplexes which are similar but non – chelated.
Effective Atomic Number (EAN)
Effective atomic number is the total number of electrons around the central metal ion present in a complex. It is the sum of the electrons of metal ion and the electron donated by the ligands.
EAN = Z-X+Y
Where, Z = Atomic number of themetal.
X = Number of electrons lostduring oxidation of metal to metal ion.
Y = Number of electrons donatedby the ligands.
e.g. In ferrocyanide ion [Fe(CN)6]4-
Z = Atomic Number of Fe = 26
X = Number of electrons lost due tooxidation of Fe2+ = 2
Y = Number of electrons donated by
6CN– = 6 x 2 = 12
.: EAN = 26 – 2 + 12 = 36
.: EAN of [ Fe (CN)6]4- is 36.
EAN of fewmetal ions
| metal | Complex | Z | X | Y | EAN |
| Ni | Ni(CO) | 28 | 0 | 8 | 36 |
| Fe | [Fe(CN)6]4- | 26 | 2 | 12 | 36 |
| CO | [CO(NH3)6]3+ | 27 | 3 | 12 | 36 |
| Zn | [Zn(NH3)4]2+ | 30 | 2 | 8 | 36 |
| Pt | [Pt(NH3)6]4+ | 78 | 4 | 12 | 86 |
ISOMERISM INCOORDINATION COMPOUND
Isomerism is the phenomenon incoordination compounds having same molecular formula but different arrangementof the ligands around the central metal atom or ion in the space.
A]Stereoisomerism
Stereoisomerism are those isomers which have the some position of atoms or groups but they differ in the spatial arrangement around the central atom.
Types of Stereoisomerism
I] Geometrical isomerism
Application Of Coordination Compounds Class 12
II] Optical Isomerism
I]Geometrical isomerism
- Geometrical isomerism is due to difference in the spatial arrangement of atoms or groups of atoms around the central metal atom or ion.
- This occurs in heteroleptic complexes.
- When two similar groups (ligands) occupy adjacent positions, the isomer is called CiS and when two similar groups are arranged opposite to one another, the isomer is called trans.
e.g. [Pt(NH3)2Cl2]
- It is present in square planner complexes with coordinationnumber 4 and octahedral complexes with coordination number 6.
1] Square Planner Complexes : [Ma2b2]n±, [Ma2bc]n±, [Mabcd]n±, [M(AB)2]n±.
2] Octahedral complexes : [Ma4b2]n±, [Ma3b3]n±, [M(AA)2a2]n±]
- Tetrahedral complexes with coordination number 4 do not showthis isomerism as all the four position are equivalent.
II] Optical Isomerism
- Optical isomerism in coordination compounds arises out ofchirality of coordination entity.
- Chiral compounds do not have any element of symmetry and areoptically active.
- Optical isomerism are non-super imposable mirror images ofeach other and are called enantiomers.
- Optical isomers rotate the plane of plane polarized light.The isomers which rotates the plane of plane polarized light in the clockwisedirection is known as d – form.
- The isomers which rotate the plane of plane polarized lightin the anti – clockwise direction is known as l – form.
- Optical isomerism is not so common in tetrahedral and squareplaner complexes. However octahedral complexes bidentate ligands show opticalisomerism. Some examples of such type of octahedral complexes are as follows:
[M(AA3)]n±,[M(AA)2a2]n±, [M(AA)2ab]n±
B]Structural Isomerism
There are four type of structuralisomerism
I] Ionizationisomerism
Thecompound which have same molecular formula but give different ions in solutionare called ionization isomers and the phenomenon is called ionization isomerism.
e.g. [CO(NH3)5SO4]Br (Red violet)
[CO(NH3)5Br]SO4 (Red)
II] LinkageIsomerism
Thecompounds which have the same formula but differ in the linkage of a ligand tothe metal atom or ion are called linkage isomers and this phenomenon is called linkage isomerism.
e.g. [ CO(NH3)5(NO2)]Cl2 (Yellow)
[ CO(NH3)5(ONO)]Cl2 (Red)
III]Coordination Isomerism
Thecompounds which have same molecular formula but different complex ionsinvolving interchange of ligands between cationic and anionic entities ofdifferent metal ions are called coordination isomers and the phenomenon iscalled coordination isomerism.
e.g. [CO(NH3)6][Cr(CN)6] and [Cr(NH3)6][CO(CN)6]
IV] HydrateIsomerism
Compoundswhich have the same composition but differ in the number of water moleculespresent as ligands and as free water molecule in the crystal lattice are calledhydrate isomers.
e.g. [Cr(H2O)6]Cl3 (Violet)
[Cr(H2O)5Cl]Cl2.H2O (Blue Green)
[Cr(H2O)4Cl2]Cl.2H2O (Green)
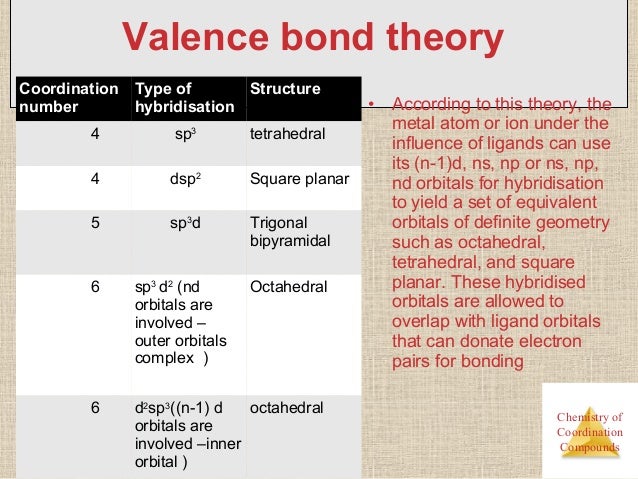
VALENCE BONDTHEORY (VBT)
Thistheory accounts for coordinate bond formation due to the overlap of vacanthybrid orbital’s of the central metal atom/ion with the filled orbital of theligands each containing lone pair of electrons.
Salientfeatures of VBT
- A central metal ionpresent in a complex provides a definite number of vacant orbital’s S, P, and dfor the formation of coordination bonds with the ligands.
- The number of vacant orbital’sprovided by the central ion is the same as its coordination number.
- These vacant orbital’sundergo hybridization to form same number of hybrid orbital’s.
- Each ligands has at leastone orbital containing a lone pair of electron.
- The geometrical shape ofthe complex ion depends upon the hybridization of the metal orbital’s.
- The vacant hybrid orbitalof the metal ion overlaps with filled orbital’s of the ligands to formcoordinate bond between metal and ligand.
- The coordination bond isstronger if the overlapping between the orbital is greater.
- If central metal atomcontains then it exhibits paramagnetic property and if central metal atomcontains no unpaired electron then, it exhibits diamagnetic property.
Structure ofcomplex compounds based on valence bond theory
- [Ni(CO)4]
- [Ni(Cl)4]2-
- [Ni(CN4)]2-
- [CO(NH3)6]3+
- [COF6]3-
Limitationof valence bond theory
- It cannot explain the spectral properties (colors) of complex compounds.
- It cannot explain why some complexes are inner complexes for the some are outer complexes for the same metal ion in the same oxidation state.
- It does not provided quantitative interpretations of thermodynamic or kinetic stabilities of coordination compounds.
- There is no distinction between weak field and strong field ligands.
- It cannot predict if a 4 coordination complex will have tetrahedral or square planar geometry.
- It does not explain the order of reactivity of inner orbital inert complexes of d3, d4, d5 and d6 ions.
- It does not explain magnetic moments.
Crystal Field Theory (CFT)
Application Of Coordination Compounds
This theory assumes that theinteraction between the metal ion and ligand is purely electrostatic. Whenligands approach central metal atom or ion the five degenerate d – orbital’s ofthe central metal atom split up into level of different energy under influenceof electrostatic field of ligands.
Salientfeatures of crystal field theory (CFT)
Application Of Coordination Compounds In Biological System
- In a complex the centralmetal atom or ion is surrounded by various atom or group of atoms calledligands.
- The ligands are either negativelycharged ion (Fˉ, Clˉ, CNˉ etc.) or neutralmolecules possessing lone pair of electrons (H2O, NH3,etc). In the neutral ligands the most electronegative atom points towardscentral metal ion.
- Ligands and metal ions actas point charges. Electrostatic interaction is present between electrons ofmetal and ligand.
- When the ligand approachthe metal atom, the electron of the central atom and those of ligands repeleach other. These repulsive forces destroy the degeneracy of d – orbital’s andsplit them into two groups called t2g and eg group.
- Electrons first occupylower energy t2g level. Electron occupancy is in accordance with Hund’srule.
- Crystal field theory doesnot account for covalent character as orbital overlap is not considered.
- Crystal fieldstabilization energy (CFSE) determines the stability of complex nature. Numberof ligands and geometry of the complex determines the magnitude of CFSE.
Weak field ligand and strong fieldligand
The ligands with small value ofcrystal field stability energy (CFST) are called weak field ligands.
The ligands with large value ofcrystal field stability energy (CFST) are called strong field ligands.
Spectrochemical series
- The arrangement of ligands in order of their increasing fieldstrengths, i.e. increasing crystal field splitting energy (CFSE) values iscalled spectrochemical series.
- Spectroscopy is used to determine the values of pairingenergy (P) and Δᵒ.
- The spectrochemical series is shown below
Iˉ ˂ Brˉ ˂ SCNˉ ˂ Clˉ˂ S2ˉ ˂ Fˉ ˂ OHˉ ˂ C2O42ˉ˂ H2O ˂ NCSˉ ˂ EDTA ˂ NH3 ˂en ˂ CNˉ˂ CO.
Limitation of crystal field theory(CFT)
- It explain only about thecentral metal ion with d orbital’s but does explain about orbital’s like s andp.
- The theory does notexplains about ∏ bonding in complexes.
- In the spectrochemicalseries water is a stronger ligand than OHˉ which is not explainsatisfactorily.
- According to this theorymetal ligand bond is ionic, but cannot explain partly covalent nature of themetal – ligand bond.
COLOURS IN COORDINATION COMPOUNDS
- The crystal field theory explains the origin of colour of coordination compounds. Colour is due to d-d transition.
- When complex compound absorb light of particular wavelength, an electron is excited from lower t2g level to higher eg level. Due to this the coordination compound has a complementary colour.
- An octahedral complex [Ti(H2O)6]3+ with d1 configuration with one electron in lower t2g level. Complex absorbs light in the blue green region. The electron is excited from lower t2g level to higher eg level.
- The colour observed is purple colour.
- [Cu(H2O)6]+2 absorbs redlight and the observed colour is blue. This is due to transition of one unpairedelectron of d9 configuration.
Relation between complex entity andwavelength
| Coordination entity | Wavelength of light absorb | Colour of light absorb | Colour of coordination entity |
| [Ti(H2O)6]3+ | 498 | Blue green | Purple |
| [CO(CN)6]3- | 310 | Ultraviolet | Pale yellow |
| [COCl(NH3)5]2+ | 535 | Yellow | Violet |
| [CO(NH3)5(H2O)]3+ | 500 | Blue green | Red/purple |
| [CO(NH3)6]3+ | 475 | Blue | Yellow orange |
| [Cu(H2O)4]2+ | 600 | Red | blue |
BONDING INMETAL CARBONYL
Metal carbonyls are the organometalliccompounds in which carbon monoxide (CO) act as the ligand.
- There is donation of lonepair of electrons of carbon (of Co) into the suitable empty orbital of themetal atom. This is a dative overlap and forms a sigma M←C bond.
- There is ∏ – overlapinvolving donation of electrons from filled metal d – orbital’s into vacantantibonding ∏* molecular orbital’s of CO. this result into theformation of M→C∏ bond.
STABILITY OF COORDINATION COMPOUNDS
- The stability of coordinationcompounds depend on the metal ion and ligands. For thermodynamically stablecompounds, this interaction is strong.
- The thermodynamic stability isquantitavely expressed in terms of the stability constant which is anequilibrium constant. For the equilibrium between metal ion and ligand.
Ma+nLx- ↔ [MLn]b+
Where,Ma+ , Lx- and [MLn]b+ are the metalion, the ligand and complex respectively and a+, x- and b+ are charges.
- The equilibrium constant forstability constant is expressed as
K= [MLn]b+ / [ Ma+][Lx-]n
- High value of stability constantindicates greater thermodynamic stability.
Stabilityconstant for formation of few complex
| System | Stability Constant K |
| Ag+ + 2NH3 ↔ [Ag(NH3)2]+ | 1.6 X 107 |
| Ag+ + 2CN ↔ [Ag(CN)2]– | 5.5 X 1018 |
| Cu2+ + 4NH3 ↔ [Cu(NH3)4]2+ | 4.5 X 1011 |
| Cu2+ + 4CN– ↔ [Cu(CN)4]2- | 2.0 X 1027 |
Coordination Compounds Chemistry
APPLICATION OF COORDINATION COMPOUNDS
There are many application of coordination compounds in different areas. Coordination compounds are used in –
- Extraction of metal.
- Analytical chemistry.
- Medicine.
- Electroplating.
- Estimation of hardness ofwater.
- Modifying the redoxbehavior of metal ions.
- Biological system.
How To Name Coordination Compounds
Join us on our social media pages- Facebook Page, Facebook Group, Twitter, Pinterest.
Application Of Coordination Compounds In Laboratory
AP Chemistry Home Page > Winter Break Project 14-15 > Chapter 23: Transition Metals Chemistry and Coordination Compounds > 23.7 Applications of Coordination Compounds
|